

FDA-AUTHORIZED FOR EMERGENCY USE
The US Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for the use of GOHIBIC® (vilobelimab) for the treatment of COVID‑19 in hospitalized adults when initiated within 48 hours of receiving IMV or ECMO. GOHIBIC is not FDA approved for this use.1
FDA EMERGENCY USE AUTHORIZATION MATERIALS:
GOHIBIC® (vilobelimab) has not been approved but has been authorized for emergency use by the FDA under an EUA for the treatment of COVID‑19 in hospitalized adults when initiated within 48 hours of receiving IMV or ECMO.1,2
The emergency use of GOHIBIC is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biological products during the COVID‑19 pandemic
under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization revoked sooner.2
What is Covid-19?
COVID‑19 is caused by a virus called a coronavirus. Patients can get COVID‑19 through contact with another person who has the virus. Patients can learn more about COVID‑19 by visiting www.cdc.gov/COVID19 or contacting their local or state public health department.3
WHAT IS GOHIBIC® (vilobelimab)?
GOHIBIC is a recombinant chimeric monoclonal IgG4 antibody that specifically binds to the soluble human complement split product C5a after cleavage from C5 to block its interaction with the C5a receptor, both of which are components of the complement system thought to contribute to inflammation and worsening of COVID‑19. GOHIBIC is not FDA-approved for any indication, including for the treatment of COVID‑19.2
DOSAGE AND PREPARATION OF GOHIBIC
The recommended dosage of GOHIBIC for the treatment of adults with COVID‑19 is 800 mg administered by intravenous infusion after dilution for a maximum of 6 (six) doses over the treatment period as described below.1
EVERY MINUTE MATTERS.
THE ICU REQUIRES EMERGENT ACTION.
ONCE YOUR PATIENT IS INTUBATED, GOHIBIC MUST BE INITIATED WITHIN 48 HOURS.
STOCK GOHIBIC NOW.
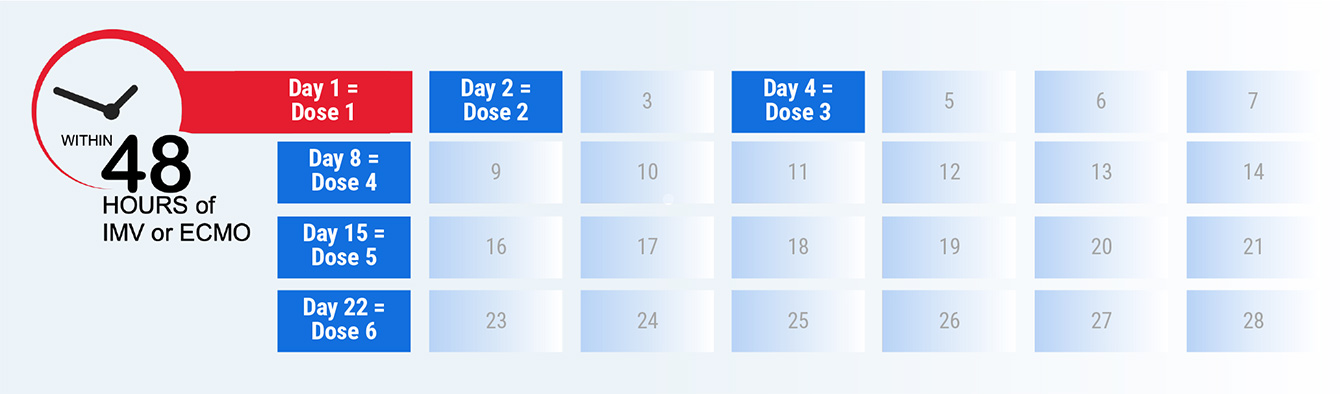
Start treatment within 48 hours of the patient receiving IMV or ECMO, followed by administration of GOHIBIC on days 2, 4, 8, 15, and 22 while the patient is hospitalized (even if discharged from the ICU)
Using aseptic technique, dilute and prepare GOHIBIC for intravenous infusion before administration. For the recommended dose of 800 mg GOHIBIC, dilute 80 mL of GOHIBIC in 170 mL of 0.9% Sodium Chloride at room temperature. Use a 250 mL infusion bag of 0.9% Sodium Chloride solution USP and follow the steps below:
- Withdraw 80 mL of 0.9% Sodium Chloride solution USP from the infusion bag and discard
- Withdraw the 80 mL of GOHIBIC from the vials and add slowly to the 0.9% Sodium Chloride solution USP infusion bag to a final concentration of 3.2 mg/mL
- To mix the solution, gently invert the bag to avoid foaming
ADMINISTRATION AND STORAGE OF GOHIBIC
Visually inspect for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if discoloration or visible particles are present. Administer diluted GOHIBIC via intravenous infusion over 30‑60 minutes. Avoid concomitant administration of GOHIBIC with other drugs in the same intravenous line.1
| Diluted GOHIBIC stored at room temperature 20°C to 25°C (68°F to 77°F) |
Diluted GOHIBIC stored under refrigeration 2°C to 8°C (36°F to 46°F) |
|---|---|
| Must be used within 4 hours. | Must be used within 24 hours and left to acclimatize to room temperature prior to administration. |
| Diluted GOHIBIC stored at room temperature 20°C to 25°C (68°F to 77°F) |
Must be used within 4 hours. |
|---|---|
| Diluted GOHIBIC stored under refrigeration 2°C to 8°C (36°F to 46°F) |
Must be used within 24 hours and left to acclimatize to room temperature prior to administration. |
PHARMACY AND HOSPITAL ACCESS
To receive copies of the GOHIBIC Frequently Asked Questions and the Academy of Managed Care Pharmacy (AMCP) Dossier, email your name, NPI number, and state of licensure to info.gohibic@inflarx.com
How to Order GOHIBIC
VERIFY ACCOUNT/ACCOUNT SETUP
Verify that your facility has an active account by contacting ASD:
ORDERING PORTAL
To access GOHIBIC for your hospital, visit the ABC Order I AmerisourceBergen portal, email, or call.
ORDERING FROM ASD HEALTHCARE | AMERISOURCE BERGEN
Important Ordering Information: CONSIDER STOCKING THE FIRST FOUR DOSES OF GOHIBIC.
| GOHIBIC NDC Code | Label Name | Generic Name | Product Description |
|---|---|---|---|
| 83000-0110-04 | GOHIBIC® (vilobelimab) Injection | vilobelimab | GOHIBIC 200 mg/20 mL (10 mg/mL) is a clear to slightly opalescent, colorless solution that is supplied in a single‑dose vial for intravenous administration after dilution. |
| GOHIBIC NDC Code | 83000-0110-04 |
|---|---|
| Label Name | GOHIBIC® (vilobelimab) Injection |
| Generic Name | vilobelimab |
| Product Description | GOHIBIC 200 mg/20 mL (10 mg/mL) is a clear to slightly opalescent, colorless solution that is supplied on a single‑dose vial for intravenous administration after dilution. |
CUSTOMER SERVICE
If you have questions about the procurement of GOHIBIC, please contact Amerisource Bergen.
Hours of operation: Monday-Thursday: 7:00am-6:30pm CT; Friday: 7:00am-6:00pm CT
Contact us
If you have questions about GOHIBIC, please contact InflaRx via phone or email.
Phone: 1-888-254-0602
General Inquiries: Info.Gohibic@inflarx.com
Medical Inquiries: medinfo@inflarx.com
AUTHORIZED USE FOR GOHIBIC
The US Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for the use of GOHIBIC® (vilobelimab) for the treatment of COVID‑19 in hospitalized adults when initiated within 48 hours of receiving IMV or ECMO. GOHIBIC is not FDA approved for this use.1
IMPORTANT SAFETY INFORMATION
Contraindications: No contraindications have been identified based on limited available data on emergency use of GOHIBIC authorized under this EUA.
Warnings and Precautions: There are limited clinical data available for GOHIBIC. Serious and unexpected adverse events (AEs) may occur that have not been previously reported with GOHIBIC use.
Serious Infections: Serious infections due to bacterial, fungal, and viral pathogens have been reported in patients with COVID‑19 receiving GOHIBIC. In patients with COVID‑19, monitor for signs and symptoms of new infections during and after treatment with GOHIBIC. There is limited information regarding the use of GOHIBIC in patients with COVID‑19 and concomitant active serious infections. The risks and benefits of treatment with GOHIBIC in COVID‑19 patients with other concurrent infections should be considered.
Hypersensitivity Reactions: Hypersensitivity reactions have been observed with GOHIBIC. If a severe hypersensitivity reaction occurs, administration of GOHIBIC should be discontinued and appropriate therapy initiated.
Adverse Reactions: The most common adverse reactions (adverse events reported with incidence ≥3% and >1% more commonly observed than in the placebo arm) are pneumonia, sepsis, delirium, pulmonary embolism, hypertension, pneumothorax, deep vein thrombosis, herpes simplex, enterococcal infection, bronchopulmonary aspergillosis, hepatic enzyme increased, urinary tract infection, hypoxia, thrombocytopenia, pneumomediastinum, respiratory tract infection, supraventricular tachycardia, constipation, and rash.
Use in Specific Populations
Pregnancy
There are no available data on GOHIBIC use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. Placental transfer of monoclonal antibodies such as GOHIBIC is greater during the third trimester of pregnancy; therefore, potential effects on a fetus are likely to be greater during the third trimester of pregnancy. In an enhanced pre- and post-natal (ePPND) study conducted in cynomolgus monkeys, placental transport of GOHIBIC was observed but there was no evidence of fetal harm following intravenous administration of GOHIBIC throughout pregnancy at doses 2.5 times the maximum recommended human dose (MRHD) of 800 mg on a mg/kg basis (see Data). The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk for major birth defects and miscarriage in clinical recognized pregnancies are 2%–4% and 15%–20%, respectively.
Pediatric Use
GOHIBIC is not authorized or approved for the emergency use in pediatric patients for the treatment of coronavirus disease 2019 (COVID‑19) in hospitalized patients when initiated within 48 hours of receiving invasive mechanical ventilation (IMV), or extracorporeal membrane oxygenation (ECMO).
Geriatric Use
Of the total number of GOHIBIC-treated patients in clinical studies for COVID‑19 receiving invasive mechanical ventilation (IMV), or extracorporeal membrane oxygenation (ECMO), 53 (30%) were >65 years. No overall differences in effectiveness or safety of GOHIBIC have been observed between patients 65 years of age and older and younger adult patients.
For additional information, please see:
Required Reporting for Serious Adverse Events and Medication Errors
The prescribing healthcare provider and/or the provider’s designee is/are responsible for mandatory reporting of all serious adverse events (SAEs) and medication errors potentially related to GOHIBIC within 7 calendar days from the healthcare provider’s awareness of the event.
To report adverse events and medication errors, complete and submit FDA Form 3500 to MedWatch online at https://www.fda.gov/medwatch/report.htm, or download FDA Form 3500 at https://www.fda.gov/media/76299/download and mail the completed form to MedWatch at 5600 Fishers Lane, Rockville, MD 20852-9787 or fax it to 1-800-FDA-0178. You may also request a reporting form by calling 1-800-FDA-1088.
In addition, please provide a copy of all FDA MedWatch forms to:
InflaRx GmbH Fax: 1-866-728-2630 Email: pvusa@inflarx.de, or call InflaRx GmbH at 1-888-254-0602 to report AEs.
IMPORTANT TO NOTE: Submitted reports must state, “GOHIBIC use for COVID‑19 under Emergency Use Authorization” at the beginning of the question “Describe Event” for further analysis. A copy of the completed FDA Form 3500 must also be provided to InflaRx per the instructions in the authorized labeling.
References: 1. GOHIBIC Fact Sheet for Healthcare Providers, InflaRx GmbH. May 2023. Available at: https://www.fda.gov/media/166824/download. 2. Emergency Use Authorization (EUA) for Vilobelimab Letter of Authorization InflaRx GmbH. April 2023. Available at: EUA 118 InflaRx GOHIBIC LOA (04122023) (fda.gov). 3. GOHIBIC Fact Sheet for Patients and Caregivers, InflaRx GmbH. April 2023. Available at https://www.fda.gov/media/166821/download.






